This is the full text of a very dense little article with a lot of gold embedded in it:
Mitochondrial reactive oxygen species drive proinflammatory cytokine production
Mitochondria are an appropriate fascination for any science writer who covers neurology, immunology, inflammation, or intracellular mechanisms — so here’s the BioWizardry primer on mitochondria.
Good primer from this article, in sci-speak:
“Mitochondria generate ATP through aerobic respiration, whereby glucose, pyruvate, and NADH are oxidized, thus generating ROS [Reactive Oxygen Species, or free radicals] as a byproduct. In normal circumstances, the deleterious effects caused by the highly reactive nature of ROS are balanced by the presence of antioxidants, including glutathione, carotenoids, and antioxidant enzymes such as catalase and glutathione peroxidase.”
Here’s a bit more insight into these fabulous little factories, gleaned from this article (which otherwise focuses on the mechanisms of a rare condition called TRAPS.)
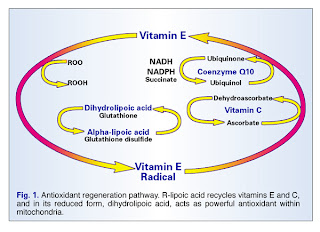
The reason for the antioxidants we take so much of to support our overloaded nervous systems is, they act specifically against reactive oxygen species, ROS. ROS are chemically reactive molecules which contain oxygen, and use the oxygen to wreak a certain amount of biological havoc. As the BioWizardry mitochondria primer mentions, mitochondria are the biggest consumers and the biggest producers of antioxidants, and the nerve and muscle cells have the biggest population of mitochondria. Therefore, when your nervous system is under siege, as in chronic pain or anxiety or lupus or MCS or what-have-you, your body may need far more antioxidants than you can get in your food to support all those mitochondria, so they can keep making energy for your cells to use in their work.
“High levels of reactive oxygen species (ROS) are observed in chronic human diseases such as neurodegeneration, Crohn’s disease, and cancer. In addition to the presence of oxidative stress, these diseases are also characterized by deregulated inflammatory responses, including but not limited to proinflammatory cytokine production. New work exploring the mechanisms linking ROS and inflammation find that ROS derived from mitochondria act as signaltransducing molecules that provoke the up-regulation of inflammatory cytokine subsets via distinct molecular pathways.”
OK, so, the mitochondria (the biggest producers and the biggest users of antioxidants) generate a specific set of ROS which trigger inflammatory cytokines.
That means, pissed-off mitochondrial cells trigger pain.
Mitochondria get pissed off by being damaged and not being able to clean themselves up:
“Mitophagy is a specialized form of autophagy that refers to the specific catabolism of mitochondria. Pharmacological inhibition of autophagy by treatment of macrophages with 3-methyladenine resulted in the accumulation of damaged mitochondria and an increase in the net amount of mtROS…”
(Autophagy is the word for when the damaged/unhealthy cell consumes itself so the damage is cleaned up and their contents get recycled for healthy cells. Cells are all about the greater good. When they’re too damaged to do their jobs, they recycle themselves.)
AND this happens whether autophagy is prevented upstream or midstream:
“Thus, autophagy regulates baseline mtROS production from individual mitochondria by a yet to be identified mechanism.”
Mind you, only so many mitochondria can autophage at a time. When damage exceeds the cell’s ability to keep up with the housework, you have a lot of damaged mitochondria.
So, take care of those mitochondria.
More on antioxidants will be coming soon. Naturally, it’s not as simple as it looks.